Fully-connected continuous manufacturing™ (EnzeneX™)
Unlocking higher yields across a range of biologics at significantly lower costs to you, our patented fully-connected continuous manufacturing™ (FCCM™) platform represents the pinnacle of our ongoing commitment to innovation.

What is EnzeneX™?
EnzeneX™ is an advanced fully-connected continuous manufacturing™ platform that harnesses the power of intensified perfusion using alternate tangential flow (ATF) and automated multi-column chromatography to achieve continuous production of biologics.
Designed for efficiency and cost-effectiveness, EnzeneX™ enables uninterrupted loading and processing of materials, delivering superior product quality and manufacturing outcomes.
What can EnzeneX™ do for you?
High-quality production
EnzeneX™ minimizes product contact with cell culture fluid, ensuring high-quality manufacturing. This is crucial for biologics that are prone to degradation including less stable and difficult-to-express proteins.
Cost-effective manufacturing
EnzeneX™ reduces processing costs by approximately 40%, providing you with cost-effective manufacturing. This is achieved with a lower cost of goods and efficient use of resources.
Adaptable design
EnzeneX™ provides flexibility with a minimum 30-50L supply.
The modular and variable bioreactors enable scale-on and scale-out flexibility, allowing you to adapt to changing production needs and optimize your manufacturing capacity.
Increased productivity
EnzeneX™ increases your manufacturing productivity, achieving approximately 10 times higher productivity upstream and 25-50% improvement downstream.
Reduced emissions
EnzeneX™ increases sustainability by reducing the equipment footprint and carbon footprint of manufacturing processes.
How does EnzeneX™ work?
Discover the science behind our patented and fully validated fully-connected continuous manufacturing™.
EnzeneX™ has enabled us to successfully manufacture commercial monoclonal antibodies (mAbs) and convert biologics from fed-batch production to FCCM™.
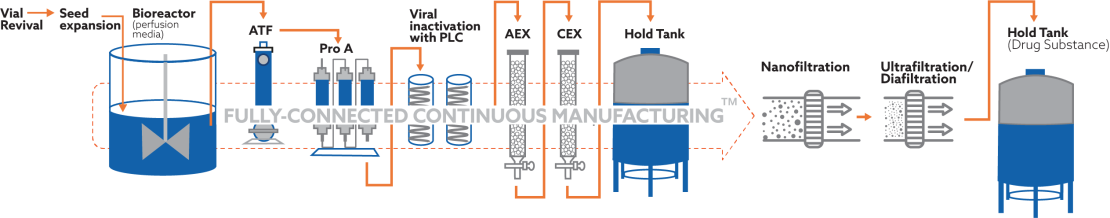
Uncover the unique advantages of EnzeneX™
Fully-connected continuous manufacturing™
- EnzeneX™ utilizes a patented combination of intensified perfusion and multi-column chromatography, ensuring the highest quality, efficiency, and cost-effectiveness in manufacturing
- This innovative process enables consistent and reliable production of biopharmaceutical products
Primed for product scaling
- EnzeneX™ offers scalability options, allowing you to transition from small-scale production to commercial manufacturing
- Two scaling options are available:
- Scale-on: Uses the same-size bioreactors with a higher process duration
- Scale-out: Involves multiple same-size suites, providing increased capacity
Designed with flexibility in mind
- EnzeneX™ prioritizes flexibility, enabling you to adapt to the changing demands of your biologics products
- This offers lower costs when scaling up or down production and improved sustainability by reducing carbon emissions and facility footprints
Join the EnzeneX™ revolution
Fueled by our continuous innovation and armed with EnzeneX™, our fully-integrated CDMO solutions and our biosimilars pipeline are designed to help bring your biologics innovations to life.
